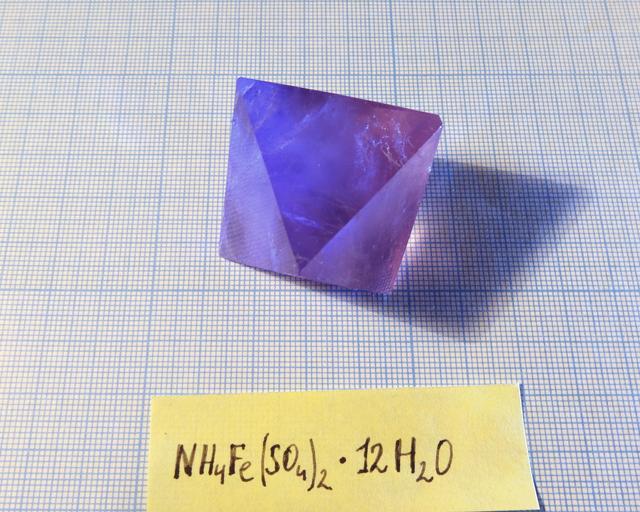
Ferric Alum
Ammonium Iron (III) sulfate, formula: NH4Fe(SO4)2·12H2O
 Double sulfate of Iron (III) and ammonium is also known as ferric alum. Despite its name, it contains no aluminum. Here, "alum" points to the wide class of double salts of similar composition: MIMII(SO4)2·12H2O, where MI is an univalent ion, usually K or NH4, and MII is trivalent: Al, Fe, Cr.
Double sulfate of Iron (III) and ammonium is also known as ferric alum. Despite its name, it contains no aluminum. Here, "alum" points to the wide class of double salts of similar composition: MIMII(SO4)2·12H2O, where MI is an univalent ion, usually K or NH4, and MII is trivalent: Al, Fe, Cr.
Properties
Crystal system: isometric (cubic)
Crystal shape: octahedron
Color: light violet. Smaller crystals are almost colorless, in big specimens coloration is much deeper.
Stability on air: not stable. Exposed to dry air, crystals dehydrate quickly, losing color and transparency in less than one day.
Protecting and storing
There are several ways to store it for long time: 1. Store crystal in a tightly closed container. To stabilize humidity level in the container, put in the same container a piece of a tissue, soaked in the same solution used to grow crystal. 2. Store it under oil or some other hydrophobic liquid. This is probably one of the most reliable methods, but it makes crystals hardly visible in the oil because of small difference in refraction ratio. 3. Cover the crystal with some protective layer. Some people use varnish, but I dislike this. There is more natural covering: grow a transparent layer of another, stable compound. It is possible if both compounds have similar crystalline structure.
With partial success, I grew a layer of ammonium alum, NH4Al(SO4)2·12H2O. Since it has much lower solubility, I first grew a layer of mixed compound, and then pure ammonium alum. Alas, layer grew unevenly and produced cracks and visible defects. Nevertheless, such protected crystal became totally stable, no changes in months.
Preparation
This compound has no everyday use, so it can only be bought in a chemicals shop. Instead, I prefer to prepare it myself.
My goal was to use only compounds, available outside of specialized stores. Thus, my preparation is surely not the easiest way to get this salt.
Source compounds
- Ferrous sulfate: FeSO4·7H2O, from the gardening shop.
- Baking soda: NaHCO3, food grade
- Household bleach: solution of NaClO, 5-15% according to labeling.
- Sulfuric acid 43%, battery acid: H2SO4
- Ammonia solution: NH3 10%, from a drug store (the most limited reagent for me).
Prepare (NH4)2SO4
Pour ammonia solution into a flask, then accurately and quickly add calculated amount of H2SO4 while stirring constantly. With 10% ammonia and 43% acid, solution becomes very hot (80 - 90 C), but not boiling. Beware sudden boiling, burns, cracking glass and NH3 fumes that are produced while reaction is not yet complete.
Prepare Fe2(SO4)3
Step 1
Dissolve FeSO4 in the minimal amount of hot water, add soda in small portions. A lot of CO2 gas is produced, beware excessive foaming. Greeninsh-gray sediment is formed, composed of ferrous carbonates and hydroxides.
Step 2
Without washing, oxidize the sediment with bleach. Add bleach in big portions (it is quite dilute). Sediment becomes brown, and after a minute, gas (CO2) is produced (I assume that FeCO3 oxidizes to Fe(III) oxides/hydroxides releasing CO2). The reaction is slow (takes hours), heat it mildly on water bath. After finishing reaction, let the sediment settle down, decant the excess solution and add new portion of bleach. Stop, when decanted liquid starts smelling chlorine. The reaction is:
2Fe2+ + NaClO → 2Fe3+ + NaCl
For me, it required 400% of calculated amount of bleach. Either my bleach was more dilute than label says, or there were side reactions, consuming hypochlorite (ClO3 formation?).
Step 3
Wash the sediment 2-3 times, remove the excess water, then add sulfuric acid (stoichiometric amount with small excess). It dissolves very badly: took 2 days. Heating on hot water bath improves it. Filter the product. You'll get dark brown solution of Fe2(SO4)3.
Prepare the double salt
Mix ammonium and ferric sulfates in equimolar amounts, let the solution evaporate (avoid strong heating, it causes hydrolysis). Ferric alum forms almost colorless crystals. Collect them and dispose the last small portion of solution that contains excess chemicals and impurities.
Then grow the crystals in the regular way. My preparation is lengthy, but it avoids uncommon reagents, hazardous gases, need to boil down large amounts of solutions and separate significant impurities.
Growing
Crystals of this compound grow easily. Note that it has very big solubility: 1240 g/L, so ensure that evaporation is not too fast. Adding small amount of sulfuric acid to the solution seem to improve quality.
Interestingly, despite the light-violet color of crystals, solution has the color of black tea.
Safety
The compound is not acutely toxic, but have irritant and corrosive properties.
More photos
Together with crystals of potassium alum