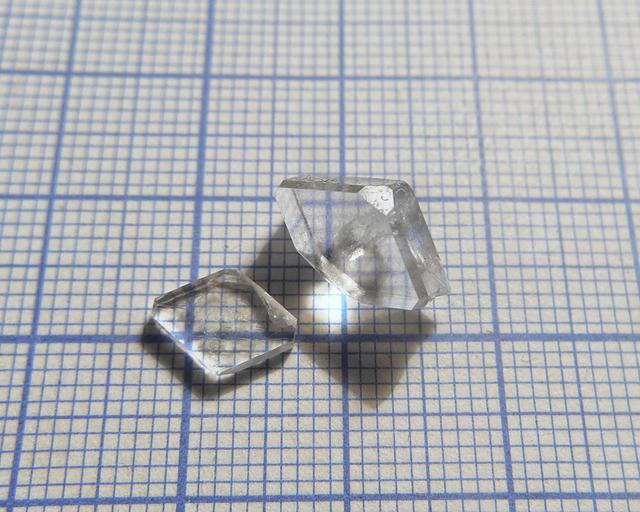
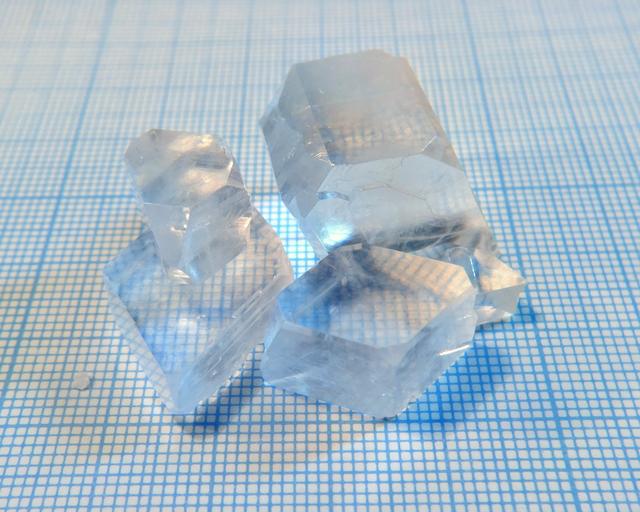
Sodium saccharin
Saccharin is the first successful chemical sugar substitute; it gained its popularity during the time of WWII and is still in use nowadays, though to a much lesser extent: there are tastier and safer substitutes now. Strictly speaking, saccharin is a weak acid, that is almost insoluble in water. Soluble saccharin is its sodium salt: sodium saccharinate.
Properties
- Crystal shape: straight prisms with rhombic base
- Color: colorless
- Stability on air: unstable, white dehydrated patches appear in several days on dry air. Should be stored in tightly closed container, better with moisture source.
Sodium displays strong birefringence (note how the line image doubles):

Its free acid also demonstrates this property.
Obtaining
Synthesis of saccharin is not easy. Probably the easiest option is to purchase some on ebay or from a chemicals provider.
Pure saccharin is not sold in the grocery stores anymore, and my local chemical providers do not sell it too. Reluctant to order it from ebay, I decided to extract it from cyclamate-based sugar substitute. Such compositions typically contain 90% of sodium cyclamate and 10% of saccharin. Cyclamate is banned in some countries, but here it is sold.
Warning: the following process is laborious, and final product is expensive, I took this way for fun. To extract saccharin, I first separated most of the cyclamate using its lower solubility:
- dissolve 100g of sweetener in 150ml of hot water,
- cool it down, separate sodium cyclamate crystals and wash them with cold water,
- boil down remaining solution and repeat crystallization/evaporation steps until the volume of solution is reduced to ~40ml.
Then I purified it using low solubility of free saccharin:
- add small excess of any acid (I used sulfuric). White sediment of free saccharine acid precipitates;
- wash the sediment, then neutralize it with soda (NaHCO3), adding it by small portions until no more gas is produced.
Resulting solution contains sodium saccharin, it can be purified by recrystallization.
This process gave me 18g of saccharin from 210g of sweetener; I managed to crystallize it almost completely.
Growing
Water solubility is high: 100g per 100 ml at room temperature. I used the usual slow evaporation setup.
Safety
The compound is safe.