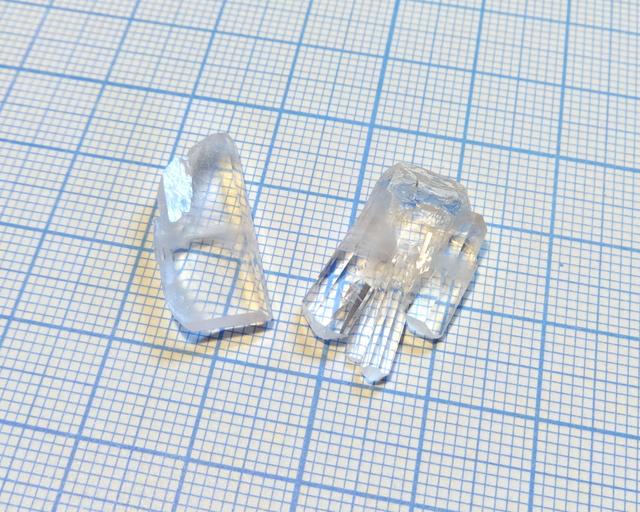
Glutamic Acid Hydrochloride
Formula: C3H5(NH2)(COOH)2·HCl, or [C3H5(NH3)+(COOH)2]Cl-.

Properties
- Crystal system: orthorombic
- Crystal appearance: transparent sticks, rectangular in plane.
- Stability on air: seem to be almost stable, but slightly lose transparency over time.
Preparation
I used 2-step preparation, starting from monosodium glutamate, sulfuric acid and hydrochloric acid.
Step 1: prepare glutamic acid
Dissolve monosodium glutamate C5H8NO4Na in water, then add stoichiometric amount of sulfuric acid. White soft sediment of glutamic acid precipitates (glutamic acid solubility in water is 0.8g/100ml ). Do not add sulfuric acid in excess, because it will dissolve the precipitate.
2C3H5(NH2)(COOH)COONa + H2SO4 → 2C3H5(NH2)(COOH)2(s) + Na2SO4
Wash the sediment with cold water.
Step 2: prepare hydrochloride
Dissolve prepared glutamic acid in stoichiometric amount of hydrochloric acid (excess is acceptable). With mild heating, white sediment quickly dissolves, giving transparent solution. The equation is:
C3H5(NH2)(COOH)2 + HCl → C3H5(NH2)(COOH)2·HCl
The solution can be immediately used for growing crystal, but better recrystallize it to get rid or Na and other impurities.
Growing
My measurements show that solubility at room temperature is around 50g/100ml. Crystals grow well with evaporation method. I added few milliliters of hydrochloric acid to the solution to compensate acid evaporation.
Safety
The compound is not toxic, but its solution is strongly acidic, it has irritant and corrosive properties, much like hydrochloric acid itself.