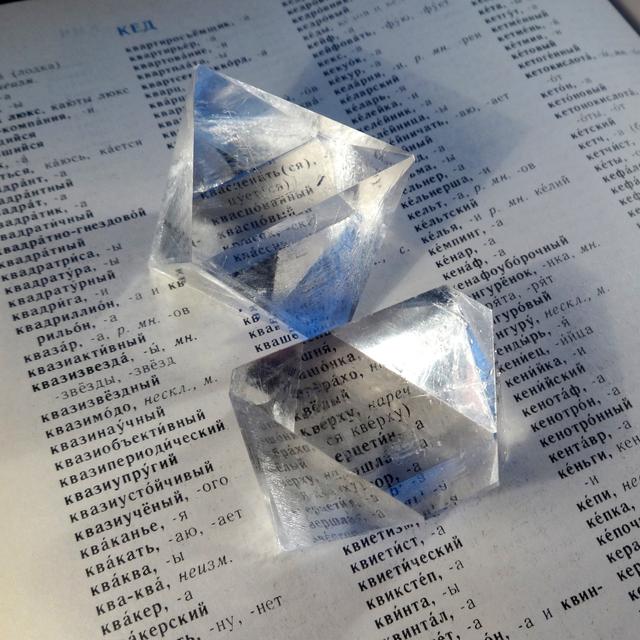
Potassium Alum
Formula: KAl(SO4)2·12H2O
 Iconic compound for crystal growing.
Iconic compound for crystal growing.
Properties
- Crystal system: isometric (cubic)
- Crystal shape: octahedrons
- Stability on air: stable
Preparation
This compound usually available in chemical shops, but can be prepared too. Since it has relatively low solubility, it will readily crystallize from any solution, containing enough of K+, Al3+, SO42- ions.
Some possible ways are:
- Dissolve Al in sulfuric acid, producing Al2(SO4)3. Reaction is very slow due to protective film on aluminum. Then add K2SO4 and crystallize.
- Dissolve Al in KOH (warning: reaction very intensive, can start boiling), getting potassium aluminate:
Al + KOH + 3H2O → K[Al(OH)4] + 3H2(g).
Then add sulfuric acid:
K[Al(OH)4] + 2H2SO4 → KAl(SO4)2 + 2H2O
Growing
I used the simplest, traditional growing method: slow evaporation.'
Prepare a glass of saturated solution, make a small seed crystal and suspend the seed on a thin thread (very fine synthetic fiber, almost invisible).
Common problems in growing alum crystals:
- Crust formation: crystalline crust grows slowly on the sides of the glass, depleting the solution and slowing down crystal growth. Solved by moving crystal to a clean glass (crust can be re-dissolved and used again).
- Mold: some molds and bacteria can thrive in concentrated alum solution, dimming the crystal and reducing evaporation. I solved it by adding a few drops of iodine tincture.
Safety
This compound is safe.