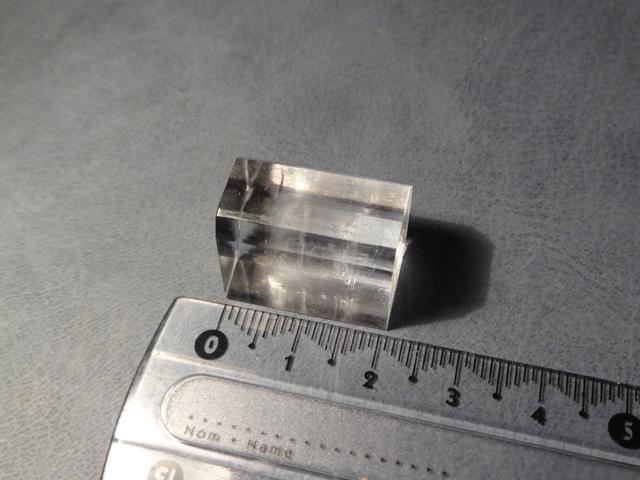
Rochelle Salt
Properties
Crystal system: monoclinic.
Stability on air: moderately stable. Over long periods of time (around year) white dehydrated spots can appear. For prolonged storage, I recommend using tightly closed ontainer. In the high humidity environments, can absorb water and liquefy.
Preparation
Rochelle salt is sold in chemical shops.
Alternatively, it can be prepared from the cream of tartar (unfortunately, it is not sold in my country, so I ordered some on eBay), and baking soda (NaHCO3).
Some sites instructions on preparation can be found on the Dom's crystal growing page (web archive link), though I've simplified that recipe by using baking soda directly, without the calcination step.
My recipe
Stir some cream of tartar powder in hot water and add small portions of baking soda until it stops fizzing. The reaction equation is:
KHC4H6O6 + NaHCO3 → KNaC4H6O6 + H2O + CO2(g)
According to this equation, per 190g of cream of tartar, 84g of soda is required.
After finishing the reaction, let the solution cool down and harvest crystallized cryst of Rochelle salt. Remaining solution can also be partially evaporated to harvest more, but don't evaporate completely: last portiono f solution contains most of the impurities.
Growing
Safety
The compound is mostly safe. In past, it was used in medicine.
More photos
References
- Wikipedia article
- My blog post
- Crystal growing site in Russian, with some gorgeous samples, much better that mine.
- My G+ gallery